Stanley A. Nasraway Jr., M.D., F.C.C.M., Department of Surgery, Tufts-New England Medical Center, Tufts University School of Medicine, Boston, Massachusetts
[Sem Resp Crit Care Med 22(2):165-174, 2001. © 2001 Thieme Medical Publishers, Inc.]
Pain and discomfort amplify the experience of fear. Pain is commonplace in every critically ill patient, not just those with wounds and surgical incisions, since a patient may be forced to lay in bed for days and weeks at a time, encumbered by all manner of indwelling catheters and hardware. Patients have an incessant compulsion to move about, shift position, and relieve pressure; yet physical weakness associated with underlying illness and the use of physical restraints will impede patient efforts, raise patient frustration, and heighten the state of agitation.
Finally, patients confined to an ICU bed for days or weeks can be expected to become sleep deprived.[3] Sleep deprivation originates from frequent interruptions of patient routine, and from continuous stimulation with light, touch, and noise. Excessive noise originates chiefly from the dozens of alarm systems that mandatorily accompany all the devices and machines associated with bedside care. These include ventilators, pulse oximeters, intravenous infusion pumps, and continuous electrocardiographic and hemodynamic monitoring. Even the many new varieties of mechanized beds, which perform lateral rotation and percussion therapy, are both noisy and alarmed. The ICU is an enormously raucous and strident place. ICU noise levels routinely exceed 80 decibels, a figure that is tantamount to a very busy urban street corner.[4] This is true for all time periods in a 24-hour day. For example, the ICU has been found to be noisier at night during the time from midnight to 0600 than at any time on a hospital ward. As a result of this bedlam, patients become progressively sleep deprived, which further augments the state of agitation, and quite often leads to paranoia or delirium. Hence, a corollary strategy in all patients, to attenuate burgeoning agitation, should be that of maximizing the length and quality of sleep.
Formal indications for sedation of the critically ill are listed in Table 1. The most important indication is simply that of anxiolysis, or the lessening of fear. This is most true for patients who are profoundly ill, yet aware enough to realize they are looking directly into the abyss of death. Sedation can mitigate the patient's sense of dyspnea, or suffocation that accompanies ventilatory failure. It is also a little-appreciated fact that sedatives can potentiate the effects of narcotics, thereby insuring better comfort and analgesia for the patient. Additionally, sedation is a mandatory prerequisite prior to, and during, co-administration of neuromuscular blockers.[7] The patient should never be subjected to the conscious sensation of paralysis; this unfortunate and avoidable cruelty is unfortunately well documented in the literature. An additional benefit of some sedatives, such as propofol and benzodiazepines, is that of amnesia; preventing unnecessary recall of the ICU with its attendant adverse experiences is a divine goal.
In short, sedation of agitated critically ill patients should start only after provision of adequate analgesia and treatment of reversible physiological causes. The use of optimal sedation, particularly in conjunction with analgesia, can reduce the risk of complications associated with the metabolic response to injury and can allow patients to better tolerate ICU care that is noxious, such as tracheal suctioning, invasive procedures, and dressing changes.
The most important advantage of propofol is its rapid onset and offset of
action (Table 2). This behavior of a "rapid on, rapid off" feature, not
available with the intravenous opiates or benzodiazepines, accounts for the
increasing popularity of propofol. Because the onset of action after a single
dose is rapid, and its effect brief (~ 10-15 minutes) due to high lipophilicity
and central nervous system penetration, propofol is given only by
continuous infusion when used for sedation. Propofol is a complex drug that
actually has three half-lives.[16] Its a
half-life, the distribution of the drug from the blood to the tissues after
intravenous administration, is very short, perhaps 2 to 3 minutes. The ß
half-life of the drug, which is basically the elimination half-life, ranges from
30 to 60 minutes. The
half-life, or terminal half-life, during which the drug is eliminated from the
third compartment, or tissue fat, ranges from 300 to 700 minutes. Clearance is
by hepatic elimination. The complexity in pharmacokinetics for propofol has
crucial implications and must be factored in when administered for prolonged
periods in critically ill patients. The large contribution of (about 50%) to the
fall of plasma levels means that after very long infusions (at steady state),
only about half the initial is needed to maintain the same plasma levels. The
large volume of distribution normally seen in the septic or injured host, in
combination with the lessened ability to clear the drug in the elderly, can
result in a prolonged recovery phase of days due to drug accumulation.[8,17] Failure to the infusion rate in patients
receiving propofol for extended periods may result in excessively high blood
concentrations of the drug. Thus, titration to clinical response and daily
evaluation of sedation levels are important during extended use of propofol in
the ICU.
The most significant adverse effect of propofol is hemodynamic destabilization. Propofol can substantially reduce cardiac output because it is both a negative inotrope and negative chronotrope. Additionally, it is a vasodilator. The combined effects on cardiac output and systemic vascular resistance can cause significant hypotension.[18] Propofol is mixed as an emulsion in a phospholipid vehicle; this additional fat source provides extra calories to the patient (1.1 kcal/mL from fat), and can cause hypertriglyceridemia.[19] Pancreatitis, perhaps related to hypertriglyceridemia, has been described after the use of propofol.[20] Accordingly, triglyceride concentrations should be routinely monitored during propofol infusions. Propofol, similar to other anesthetics, is a potent respiratory depressant, suppressing both the hypercarbic and hypoxemic drives of ventilation. This effect synergizes with that of other drugs, and is dose-dependent.
Propofol requires a dedicated intravenous catheter when administered as an infusion, due to the potential for drug incompatibility and infection. Pain with peripheral injection has been described; therefore, central delivery routes are preferred. Microbial superinfection has been described in the operating room environment,[21] but not in the ICU.[22] Concerns about bacterial contamination have led to one manufacturer's suggestion to change solutions and tubing every 12 hours; moreover, propofol solutions now come with an antibacterial preservative. Very long-term infusions (days) can result in some tolerance; more importantly, this sets the patient up for a withdrawal syndrome that includes tonic-clonic seizures, in the event the propofol infusion is not judiciously tapered.[23,24] In rare instances, the use of propofol can turn the urine, hair, and nailbeds green.[25]
All benzodiazepines exert their effect centrally by binding to a specific
high-affinity binding site in the brain, found ubiquitously in humans and all
mammalian systems. Binding to the receptor facilitates endogenous -aminobutyric acid (GABA) neurotransmitter activity, which results in
hyperpolarization of the neuron after influx of chloride ions into the
cell.[26] The hyperpolarized state increases the
threshold for and thereby prevents depolarization of the neuron, causing the
clinical state of sedation. Other sedating compounds, including alcohol and
barbiturates, act in a similar fashion.
The properties of the intravenous benzodiazepines, in comparison with propofol, are listed in Table 2. Diazepam and midazolam are noteworthy because they feature a greater lipophilicity, enabling them to cross the blood-brain barrier more quickly, as compared with lorazepam. This means that diazepam and midazolam have a much more rapid onset of action and are the appropriate choices among benzodiazepines when an immediate sedating effect is required at the bedside. Either one of these is an appropriate choice for short-term sedation lasting minutes to a few hours. Lorazepam, by contrast, has no active metabolites. Lorazepam has an intermediate duration of action; it may be a more steady and predictable agent for long-term maintenance of sedation in the chronically critically ill, both because of its lack of active metabolites and because its decreased lipophilicity yields a lesser volume of distribution and reduced time to elimination. However, it must be said that overall, the predictability of recovery from prolonged sedation would appear to be more favorable with propofol than with any of the benzodiazepines.[28-30] The competitive antagonist, flumazenil,[26] can reverse excessive central nervous system depression and other toxic effects by the benzodiazepines. Flumazenil has a very rapid onset of action and a relatively short duration of action (30-45 minutes) relative to the prolonged effects of the benzodiazepines; hence, it has been given by continuous infusion to expedite awakening as an adjunct in weaning from mechanical ventilation.[31]
In addition to erratic recovery after long-term usage, benzodiazepines can have other adverse effects. These agents cause ventilatory depression by abolishing the hypoxemic drive to ventilation. When given in conjunction with narcotics, which respectively abolish the hypercarbic drive to ventilation, it is readily understandable how the combination of these two classes of drugs can substantially depress respiration. Tolerance to the benzodiazepines may occur after prolonged therapy, and ever-increasing doses of midazolam have been reported.[32] The long-term use of these agents predisposes to a chemical dependency, and abrupt or imprudent discontinuation has been observed to cause a withdrawal syndrome, resulting in rebound agitation in the patient.[29,33,34] Hence, it is recommended to taper these agents after steady and heavy exposure for a period of days.
Opiates have not been comparatively studied in the critically ill. Morphine is the standard against which all other analgesic agents are compared. Morphine has a half-life of 2 to 3 hours after intravenous administration, but duration is prolonged in the setting of renal or hepatic dysfunction. The active metabolite morphine-6-glucuronide accumulates in renal failure, further extending the duration of action. Fentanyl is a synthetic opiate with 100 times the potency of morphine. It has a more rapid onset of action due to its greater lipophilicity; it also has a more rapid offset of action due to its shorter half-life, approximating 30 to 90 minutes. Fentanyl has a reputation for possibly inducing less hemodynamic instability, in part because it does not induce histamine release like morphine.[1,36] Therefore, fentanyl is the preferred choice of agent in patients in circulatory shock, on vasopressors, due both to its shorter duration of action and its lack of histamine release. Dilaudid is a more potent analgesic and sedative than morphine, with a similar duration of action. However, dilaudid has no active metabolites, has protein binding that is less than that of the other opiates, and also does not provoke histamine release.[38] For these reasons, dilaudid is an advantageous choice for the chronically critically ill patient in renal failure. All three of these agents can be given by intermittent bolus or by continuous infusion; prolonged administration for days will result in peripheral uptake and excessive accumulation.
The standard reversal agent in the setting of opiate overdose is naloxone, a competitive antagonist. The half-life of naloxone is 45 minutes, and, like flumazenil, it may need to be administered as a continuous infusion because of the prolonged duration with accumulation by the opiates.[1]
One study found sustained continuous infusions of sedatives was associated with a statistically longer duration of mechanical ventilation and length of stay.[30] However, this prospective observational study was seriously flawed in that patients were not randomized, and administration of sedatives, by intermittent bolus or by continuous infusion, was not protocolized. Perhaps a better conclusion from this study is that the nonstandardized, haphazard approach to sedative management might result in as much harm as good. The same group of investigators subsequently tested in a randomized manner the practice of protocolized sedation, intermittent or continuous, with traditional non-protocol-directed sedation administration on mechanically ventilated patients.[52] Patients randomized to protocolized sedation, either intermittent or continuous, had reduced duration of mechanical ventilation and reduced length of stay.[52]
A second study from another investigative site also found potentially prolonged duration of mechanical ventilation for patients receiving continuous infusions.[29] This study by Kress et al was a prospective randomized, controlled trial of 128 mechanically ventilated patients receiving continuous infusions of midazolam or propofol. In the intervention group, the continuous sedative infusion was interrupted until the patient awakened, whereas in the control group, the infusion was interrupted at the discretion of the clinicians or not at all. The infusion of drug was strictly protocolized, and defined endpoints were rigorously applied. The investigators found that daily interruptions of drug, so as to avoid excessive medication and toxicity, was associated with a shorter duration of mechanical ventilation ICU length of stay.[29] The authors claim there was no appreciable increase in side effects from daily awakenings for the intervention group, although discomfort and fear in the awake, critically ill patient are difficult to quantify. Closer scrutiny of the data shows the outcome differences were essentially derived from those patients receiving midazolam infusions, since total midazolam infused was almost double in the control group. In contrast, there was no difference in the total dose or average rate of propofol given between the groups. This finding again points to the findings from the aggregate literature indicating that propofol infusions are superior to benzodiazepine infusions in terms of time to awakening and better overall predictability. One could conclude that daily interruption of sedation and the potential deleterious effects therefrom could be altogether avoided if the clinician relied principally on propofol, and less on benzodiazepines, which are more erratic when given for sustained periods.
These data support the thesis that propofol is at least as efficacious as benzodiazepines for sustained delivery, and very likely more cost effective, even though propofol at the time of this writing has a higher acquisition cost than that of any of the generically available benzodiazepines. Continuous infusion delivery, when protocolized, remains a pharmacologically favorable mode and is less labor intensive than intermittent bolus delivery.
The 2-adrenergic receptor can be found in
the central and peripheral nervous systems, in the heart, and on vascular smooth
muscle.[54] The hypnotic-sedative action of the
2-adrenergic agonist is situated in the
locus ceruleus of the brain stem; whereas the analgesic properties are
effectuated at receptor sites estimated to be located somewhere in the spinal
cord. There are conflicting reports on cognitive performance by
2-adrenergic agonists; some reports indicate
modulation of spatial working memory with increased performance,[55] whereas others have reported reduced
cognition.[56] By comparison, benzodiazepines
and propofol are agents that relieve anxiety while decreasing cognitive
function; whereas neuroleptic agents (haloperidol or droperidol) blunt affect
but purportedly preserve intellectual functioning.
The 2-adrenergic agonists also exert
cardiovascular effects. Bradycardia may occur via two pathways: a vagomimetic
effect, and blockade of the cardioaccelerator nerve. Stimulation of presynaptic
2-adrenergic receptors located in the
sympathetic nerve endings inhibits the release of norepinephrine; activation of
postsynaptic receptors by
2-adrenergic agonists in the central nervous system
leads to inhibition of sympathetic activity. In sum, this sympatholytic effect
can result in regional vasodilatation and hypotension.[54,57] In some cases, however, hypotension may be
offset by
2-adrenergic agonists' direct vascular
smooth muscle stimulation, leading to vasoconstriction. Other poorly understood
effects of this class of agents include anti-shivering and diuretic actions.
Amnesia is a desirable quality when treating patients who must endure the
adversity of the ICU environment; unfortunately, the amnestic property of
2-adrenergic agonists is relatively weaker than those
produced by benzodiazepines or by propofol.[57]
In one study, a small number of patients receiving dexmedetomidine were observed
to express resentment for the increased state of awareness and subsequent stress
they sustained while in the ICU.[57]
The essential features of dexmedetomidine are depicted in Table 3.
Dexmedetomidine is more potent than clonidine, centrally, in that it has an
eightfold greater affinity for the 2-adrenoceptor. Its effects are predictable and
dose-dependent. The major complications are hypotension and bradycardia, which
may occur in upwards of 30% of recipients.[57,58] This may be particularly true in the
hypovolemic, already vasoconstricted patient, where greater prudence would be
required during administration. On the other hand, this is no less true when
administering other agents, such as propofol. Of note, a selective
2-adrenoceptor antagonist has been described for the
reversal of excessive sedation or hypotension.[59] Unlike etomidate, dexmedetomidine has negligible
effects on adrenal steroidogenesis in mammals.[60] The use of
2-adrenergic agonists in varying postoperative patient
populations is well documented, as are multiple delivery routes (e.g.,
intravenous, intercostal, and epidural).[61]
Employing (
2-adrenergic agonists attenuates the
problems of narcotics, such as ileus, urinary retention, and ventilatory
depression and abuse liability.
Dexmedetomidine has been shown widely to be clinically effective for short-term sedation and analgesia, either by itself or in combination with opiates and benzodiazepines.[57,62,63] Two randomized, double-blind, parallel, placebo-controlled, multicenter studies were conducted to evaluate the safety and efficacy of dexmedetomidine in intubated patients (n = 754) who were mechanically ventilated in the ICU.[62,63] The initial dose and maintenance infusion (see Table 3) were titrated to achieve mild sedation, with patient arousal to verbal commands. Treatment started within 1 hour of admission to the ICU and continued for at least 6 hours after extubation (maximum infusion period of 24 hours). The primary outcome measure in these studies was the amount of rescue medication, in the form of midazolam or propofol, needed to maintain the specified level of sedation. A second outcome measure was the amount of supplemental morphine required to relieve pain. In both studies, approximately 60% of the dexmedetomidine recipients required no additional sedation. Supplemental propofol and midazolam requirements were reduced by 7 times and 4 times, respectively. Moreover, dexmedetomidine subjects required 50% less morphine in both studies.[62,63] A third study, out of the United Kingdom, designed very similarly, also demonstrated significantly less need for midazolam and morphine in dexmedetomidine recipients.[57]
Overall, dexmedetomidine holds superior promise as a combination sedative-analgesic agent in the ICU. One obvious advantage, in contrast to other sedation agents, is the lack of ventilatory depression. Dexmedetomidine can be administered during and after extubation from mechanical ventilation, without the need to be weaned prior to extubation to protect against depression of basal respiratory rates. Because infusion can be continued through the postextubation period, dexmedetomidine provides increased flexibility in the timing of extubation. The other chief advantage of dexmedetomidine is the reported easy arousability. Patients are calmly and easily roused from sleep, permitting better communication, and then may easily return to sleep. Complicated tasks, such as communication by pen and paper, are possible. The future of this sedative depends on understanding appropriate patient populations and circumstances surrounding its use. It has not, for example, been adequately studied for the purpose of conscious sedation, as would be necessary for invasive procedures, radiological examinations, or transport of the critically ill. Its amnestic properties need to be better elucidated. The circumstances under which co-administration of opiates would be commonly expected have not been adequately described. Inappropriate use of this agent might induce or aggravate cardiac conduction defects, or ventricular output.
The field of sedation in the ICU is still constrained by a dearth of high quality, randomized, prospective trials comparing agents, as well as comparing monitoring techniques and scoring scales.[28] Critical care clinicians have a clarion mandate to promulgate and make use of clinical practice guidelines, to integrate this information in a manner that is appropriate for their practice setting, and to establish protocols as a way of reducing practice variability and the complications that usually accompany such variation. Once this information is applied at the bedside, there is a final obligation to measure the effects of its implementation, and the ensuing consequences. We have had good success with the standardized protocol for sedation put into place in 1998 in the Surgical Intensive Care Unit at the Tufts-New England Medical Center in Boston (Figure 1). This protocol relies first on fentanyl to assure relief from discomfort, and opiate-related anxiolysis. Propofol is added when fentanyl alone is not enough. Lorazepam is added thereafter, in those rare instances when the patient is refractory to the combination of fentanyl/propofol. Sedation is titrated to the Riker Sedation-Agitation Scale,[65] based on clinical requirements.
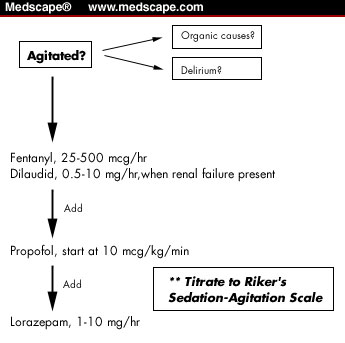
Figure 1. Simplified version of the sedation/analgesia protocol employed in the Surgical Intensive Care Unit at the Tufts-New England Medical Center in Boston, Massachusetts. The Riker Sedation-Agitation Scale is used to monitor the level of sedation.[65]One obstacle to devising standard management protocols is the daunting challenge of determining "the best practice." Controversy haunts nearly every area of conventional intervention. Yet, it isn't necessary to determine "best practice," just "sensible practice." The gains that result come not from best practice, but from the constancy of practice66 that leads to a decrease in errors, improved effectiveness, and the reduction in uncontrolled variables. Mascia and colleagues implemented protocolized guidelines for the use of sedation, analgesia, and neuromuscular blockade in their ICU, and documented subsequently improved outcomes in survival, with reductions in cost and length of stay.[67] The emerging standard of care for sedation practice in the ICU is to adopt a protocolized approach with medications titrated to specific endpoints, including a subjective monitoring scale, and to periodically measure the effects of this practice.[51,52,64]
Author's Note: All reporting and analyses were performed at the Tufts-New England Medical Center, Boston, Massachusetts.
- To attenuate fear and anxiety
- To potentiate analgesia
- To reduce metabolic demands, particularly during circulatory shock
- To facilitate tolerance to procedures, and as a chemical restraint
- As a mandatory adjunct to neuromuscular blockade
- To reduce unnecessary recall (amnesia)
- To facilitate terminal care
a For 70-kg adult
Propofol Midazolam Lorazepam Diazepam Bolus dose a 2 mg/kg b 1-5 mg 1-5 mg 2-10 mg Elimination half-life 30-60 min 1-4 h 10-20 h 20-70 h Onset 1-2 min 2-5 min 5-20 min 2-5 min Lipophilic high high moderate high Active metabolites no yes no yes Continuous IV yes yes yes no
b Represents induction dose; maintenance dose would be 5-80 mcg/kg/min.
Indication sedation, with some analgesia; Mechanism of action 2-adrenoceptor agonist
Advantages anxiolysis, analgesia, easy rousability, no respiratory depression Route of administration intravenous Usual dosage load at 1.0 µg/kg for 10 min; then 0.2 - 0.7 µg /kg/h Elimination half-life 2 hours Route of elimination 95% renal excretion Adverse events hypotension, bradycardia